by Bethany Ehlmanna
aBrown University, Providence, RI, USA
Received February 2010; accepted in revised form 16 February 2010; available online 23 March 2010
Introduction by Paul Mahaffy (NASA Goddard Space Flight Center)
The past decade of Mars missions has been a golden age of Mars exploration with a focus on the habitability of our next-door neighbor planet. With every new set of successful measurements from surface rovers or from orbit a fresh and often surprising perspective emerges on the geological and geochemical processes that brought Mars to its present cold and dry state. In the first contribution of this series (Geochemical News 141) we surveyed the recent progress from several such missions and examined the measurements we hope to make from instruments on a highly sophisticated rover (Curiosity) to be launched in 2011. With the present contribution, Bethany Ehlmann, one of the recent leaders in analyzing and interpreting Mars orbital infrared spectroscopy data will describe these techniques and results and the new view of the history of Mars that is emerging from these studies. (See NASA's illuminating animation at http://mars.jpl.nasa.gov/mro/gallery/video/)
This paper is the second in a series organized by Dr. Paul Mahaffy (Chief, Atmospheric Experiments Laboratory, Solar System Exploration Division, NASA Goddard). In the first installment, Dr. Mahaffy discussed Curiosity, the 900 kg, nuclear-powered rover scheduled to land on Mars in late 2012. He reviewed the rover's landing system and its extensive array of integrated scientific instrumentation. (http://www.geochemsoc.org/publications/geochemicalnews/gn141oct09/sampleanalysisatmars.htm).
Stay tuned for the third paper, which will describe the x-ray diffraction and x-ray fluorescence instruments on Curiosity and how they will help characterize the Martian surface.
Diverse aqueous environments during Mars' first billion years: the emerging view from orbital visible-near infrared spectroscopy
Phyllosilicates, sulfates, carbonates, zeolites, chlorides, perchlorates, and opaline silica: news of discoveries on Mars is starting to read like a mineralogy textbook. With the arrival of orbiting visible/near-infrared (VNIR) spectrometers, first in 2003 on the European Space Agency's Mars Express followed in 2006 by NASA's Mars Reconnaissance Orbiter (MRO), it is now clear that chemical and mineralogic evidence of past aqueous geochemical processes persists to the present, recorded in rocks and sediments. Coupled with data from landed missions, Mars exploration is moving beyond excitement about simply past liquid water towards understanding that ancient Mars probably hosted a diverse array of aqueous environments. Recent data suggest that hydrothermal, lacustrine, and pedogenic settings were part of the Martian landscape during its first billion years. Could one (or more) of these environments have been habitable or inhabited? Using the current data to guide the upcoming set of landed and orbital missions, we may be poised to find out. Here I review recent mineralogic findings, detail the methods, and discuss implications for understanding the first billion years of Mars history-a period in which the geologic record on Earth is sorely lacking.
Following the water...and the minerals
For the past decade, "follow the water" has been NASA's guiding principle for selection of Mars exploration missions and landing sites. The reason is simple: on Earth wherever we find water we find life. Tracing the history of this important Mars volatile may be our best means of understanding whether life could have existed there, as well as understanding the fundamental processes necessary to sustain water-rich worlds like our own through time.
Since the Mariner missions of the late 1960s, it has been known that liquid water was once a powerful force shaping Mars' surface. Since the first Mariner and Viking orbiters, orbital imaging has revealed large outflow channels, valley networks, evidence for crater lakes and, some posited, a possible northern ocean (Figure 1). Yet subsequent missions did not reveal the mineralogy and chemistry one might expect for a once watery world. Elemental composition measurements from landers and rovers showed rocks of primary volcanic composition. A few tantalizing hints of salts in Mars soils and in Mars meteorites pointed to at least small amounts of liquid water, but the basaltic rocks had not altered to clays or oxides. There appeared to be no secondary rocks, e.g. carbonates, that on Earth precipitate in large bodies of water and that should precipitate on Mars from waters in equilibrium with its 95% CO2 atmosphere.
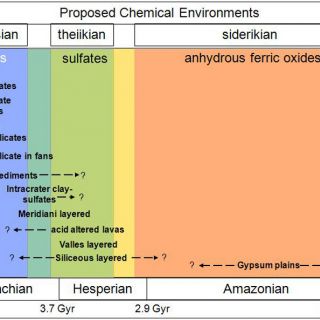
But in 2004, following the water took an exciting, chemical/mineralogical turn. The Opportunity Mars Exploration Rover discovered sulfate-rich sedimentary rocks at Meridiani Planum, most likely deposited in shallow surface waters (Squyres et al., 2004). Then the Observatoire pour l'Minéralogie l'Eau les Glaces et l'Activité (OMEGA) visible/near-infrared imaging spectrometer on Mars Express began finding hydrated minerals from orbit (Bibring et al., 2005; for methods, see ‘Identifying minerals from orbit' below). One class was the sulfate salts, such as at Meridiani, found in rocks from Mars' middle epoch, the Hesperian, approximately 2.9-3.7 Gyr. The second class was phyllosilicates, in this case Al-, Fe-, and Mg- smectite clays, found in the most ancient Noachian rocks, >3.7 Gyr. The youngest Amazonian terrains, <2.9 Gyr, had none of these hydrated minerals. This suggested a directional evolution for Mars, traceable through mineralogy (Bibring et al., 2006). A wet, circum-neutral pH clay-forming epoch ("phyllosian") was followed by a drier, more acidic sulfate-forming epoch ("theiikian"). By three billion years ago, only anhydrous alteration processes persisted over large scales ("siderikian").
The higher spatial and spectral resolution NASA follow-on to OMEGA, the Compact Reconnaissance Imaging spectrometer for Mars (CRISM; Murchie et al., 2007) on MRO has discovered many more occurrences of hydrated minerals than previously known as well as refined the detections of phyllosilicates and sulfates to reveal extraordinary mineralogic diversity within these classes as well as new mineral classes (Table 1). It is now possible to perform meters-scale geologic mapping from Mars orbit that includes both morphological and mineralogical properties of the units. By understanding the context and associations of these diverse alteration minerals, we are better able to distinguish the geological environment in which they formed. Coupled mineralogy with morphology allowed the identification of distinct chemical environments and their rough time ordering (Figure 2; Murchie et al., 2009), which we detail further below (‘Mineralogic clues to the first billion years').
Table 1. Minerals resulting from aqueous alteration, discovered on Mars through January 2010. All mineral phases are observed from orbit with VNIR spectroscopy except for phases in italics, which have been reported by other orbiting and landed missions.
| Class | Group/mineral | Formula | References |
|---|---|---|---|
| Phyllosilicates | Fe/Mg smectites (e.g., nontronite, saponite) |
(Ca, Na)0.3-0.5(Fe,Mg, Al)2-3(Si, Al)4O10(OH)2 | Poulet et al., 2005; Mustard et al., 2008 |
| Montmorillonite | (Na,Ca)0.33(Al,Mg)2(Si4O10)(OH)2 | Poulet et al., 2005; Mustard et al., 2008 |
|
| Kaolin group minerals (e.g. kaolinite, halloysite) |
Al2Si2O5(OH)4 | Bishop et al., 2008; Ehlmann et al., 2009 |
|
| Chlorite | (Mg,Fe2+)5Al(Si3Al)O10(OH)8 | Mustard et al., 2008; Ehlmann et al., 2009 |
|
| Serpentine | (Mg, Fe)3Si2O5(OH)4 | Ehlmann et al., 2009 | |
| High charge Al,K phyllosilicate (e.g. muscovite or illite) |
KAl2AlSi3O10(OH)2 | Mustard et al., 2008; Ehlmann et al., 2009 |
|
| Other hydrated silicates | Prehnite | Ca2Al(AlSi3O10)(OH)2 | Clark et al, 2008; Ehlmann et al, 2009 |
| Analcime | NaAlSi2O6 · H2O | Ehlmann et al., 2009 | |
| Opaline silica | SiO2 · H2O | Milliken et al., 2008; Ehlmann et al., 2009 |
|
| Carbonates | Magnesium carbonate (e.g. magnesite) |
MgCO3 | Ehlmann et al., 2008b |
| Calcium carbonate | CaCO3 | Phoenix lander (Boynton et al., 2009) | |
| Sulfates | Fe/Mg mono- and poly- hydrated sulfates | (Fe,Mg)SO4 · nH2O | Gendrin et al., 2005 |
| Gypsum | CaSO4 ·2H2O | Langevin et al., 2005 | |
| Alunite | KAl3(SO4)2(OH)6 | Swayze et al., 2008 | |
| Jarosite | KFe(III)3(OH)6(SO4)2 | Milliken et al., 2008 | |
| not a named mineral | FeSO4(OH) | Swayze et al., pers. comm. | |
| Chlorides | chlorides | metalCl | Thermal Emission Spectrometer (Osterloo et al., 2008) |
| Perchlorates | perchlorates | (Mg,Ca)(ClO4)2 | Phoenix Lander (Hecht et al., 2009) |
| Fe Oxides | Hematite | Fe2O3 | Christensen et al., 2000; Bibring et al., 2007 |
| Goethite | FeO(OH) | Farrand et al., 2009 |
Identifying minerals from orbit
The fundamental advance that led to the discovery of these diverse water-related minerals only recently has been the application of orbital hyperspectral, visible/near-infrared (VNIR) imaging. The 0.4-2.6 μm wavelength region has proven to be most useful for mineral identification on Mars. VNIR spectroscopy is a technique commonly employed in the laboratory that is sensitive to changes in the energy state of transition metals' outer electrons (Fe is especially important in rock-forming minerals) as well as vibrations between atoms chemically bonded in mineral structures. The electrons and vibrating molecules, respectively, absorb radiation at characteristic wavelengths leading to distinctive absorption features in reflected light spectra that are often diagnostic of specific minerals. Usually, the electronic transition absorptions are broad and occur at wavelengths <1.5 μm (Burns, 1993). The fundamental vibrations due to OH, CO3, and H2O occur at much longer wavelengths (3-15 μm), but overtones and combination tones occur in the VNIR range measured by orbiting instruments. While these are weaker than the fundamentals in transmission spectra, light at VNIR wavelengths is multiply scattered in particulate media on planetary surfaces. The result is that the overtones and combinations may sometimes be stronger than the fundamentals that occur in the thermal infrared wavelengths (Clark et al., 1990).
The imaging spectrometers collect light in two different ways. In its infrared channels, OMEGA is a whisk broom imager which scans back and forth across the surface as it passes over, collecting one pixel in 352 wavelengths (0.35-5.1 μm) at a time with a spectral sampling of 7-20nm. CRISM is a push broom imaging spectrometer which collects a single row of pixels in 544 wavelengths (0.4-4.0 μm). Both instruments build up an image, north-south or south-north along the path of their polar orbits. The end result for each observation is the same: an image cube with x and y spatial dimensions and a z spectral dimension so that the VNIR spectrum of each pixel on the surface can be interrogated separately. OMEGA's spatial resolution ranges from 300-4800m/pixel, and global coverage is nearly complete. CRISM will complete a global map of Mars at 200m/pixel and in targeted mode acquires images at up to 18m/pixel.
The raw sensor data are corrected to radiance using laboratory data and data from an onboard calibration sphere. They are then converted to illumination angle-corrected I/F, an effective reflectance, by ratioing to the solar spectrum at Mars distance and dividing by the cosine of the incidence angle of light. To investigate surface mineralogy it is necessary to correct for strong bands resulting from atmospheric absorptions. Although more complex methods exist, most commonly employed is the "volcano scan method" in which each pixel is divided by scaling an atmospheric transmission spectrum to fit the band depth of the CO2 triplet absorption at 2.0 μm. The atmospheric transmission spectrum used is derived by taking observations over the base and top of the 27-km high Olympus Mons volcano and ratioing the difference. Commonly, corrected data from an area of interest on the surface are also ratioed to corrected data from a "boring" area to eliminate residual artifacts from instrument calibration and highlight the distinctive absorption features.
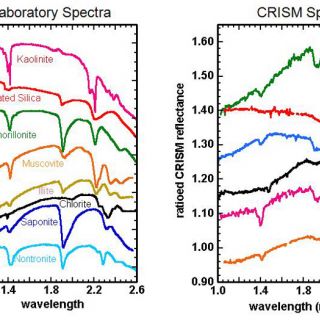
After these corrections are made, individual spectra or spectra averaged over a given region can then be compared to laboratory spectra (e.g. Clark et al., 2007; http://speclab.cr.usgs.gov/spectral.lib06/) to make mineral identifications (Figure 3 and 4). For example, in smectites, the principal absorptions are near 1.4, 1.9 and 2.2-2.3 μm. These are due to vibrations of water and OH, water, and metal-OH structural elements, respectively. Shifts in band position occur, however, when Fe- (1.43, 2.29) vs. Mg- (1.39, 2.32) vs. Al- (1.41, 2.21) is the principal octahedral cation, allowing even structurally similar phases such as nontronite, saponite, and montmorillonite to be distinguished from orbit (Clark et al., 1990; Mustard et al., 2008).
Once identified, by mapping absorption band depth (Pelkey et al., 2007) or using more sophisticated parameters to examine overall spectral shape (Clark et al., 2003), the distribution of VNIR spectral signatures diagnostic of particular minerals can be mapped spatially. The high-resolution mineralogic data can then be combined with high-resolution imaging data sets-down to 25 cm/pixel resolution with MRO's HiRISE camera-to study mineralogy in concert with morphology.
Mineralogic clues to the first billion years
As Figure 2 shows, nearly a dozen new aqueous chemical environments have been identified on Mars and are now the ongoing subject of intensive study (Murchie et al., 2009). A period of particular interest is the Noachian and the Noachian to Hesperian transition. During this period of Earth's history many important events happened: the first crust formation, the first liquid water, late heavy bombardment by meteorites, prebiotic chemistry and events leading to the origin of life. When did the Earth become habitable and when did it become inhabited? The timing and environmental conditions remain shrouded in mystery due to the recycling of the rock record by plate tectonics. Far less than 1% of Earth's rock record is from this period and much of what does exist has been heavily altered by metamorphism, weathering, and more recent biologic activity. However, about half of Mars' crust dates back to before 3.5 Gyr. Moreover, this appears to be the period where liquid water played the greatest role in planetary geologic processes. Little is known about these early conditions and a big question is why did Mars apparently begin to dry by the end of its first billion years. By studying the environments of early Mars, we gain insight into the history of water on Mars and also the earliest watery environments on terrestrial planets.
Environmental history from mineralogy
While some of the minerals on Mars form under a variety of geochemical conditions and at relatively unconstrained temperatures and pressures (e.g. smectites, chlorites, gypsum), some of the minerals form in only narrow temperature, pH or redox conditions (e.g. prehnite, analcime, alunite, jarosite). The recently discovered alteration mineral diversity suggests global Mars trends were modulated by specific local conditions. Moreover, distinctive stratigraphies and morphologies of the mineral-bearing units suggest multiple settings for liquid water. Here are a few of the earliest environments:
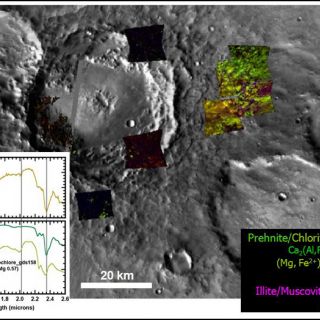
'Deep phyllosilicates': these geologic materials are the oldest altered crust on Mars and, in some areas, such as in the Nili Fossae fracture system, the basement layer of a complex stratigraphy (Figure 5). Here the basaltic materials have been partially altered to materials whose VNIR spectral signature is dominated by that of Fe/Mg smectite clays. The materials are frequently brecciated and sometimes have raised veins and fractures. In this region, these clay-bearing materials are the deepest exposed unit and apparently unaltered mafic materials cap them (Mustard et al., 2009). Elsewhere, hydrated minerals buried deeply in Mars' crust are exposed by craters drilling into the southern highlands and throwing out material. Some of the "deep phyllosilicates" have a distinctively hydrothermal character. Minerals and mineral assemblages indicative of low-grade metamorphism such as analcime, illite, and prehnite are found associated with craters. Figure 6, for example, shows a crater with prehnite- and chlorite-bearing ejecta deposits. Prehnite is a mineral diagnostic of the temperature and pressure of its formation since it only forms at temperatures between about 200-350°C and pressures <3 kbar. That it is in ejecta deposits means that it was most likely transported ballistically during impact, excavating materials from depth, rather than formed in-situ (Ehlmann et al., 2009). "Deep phyllosilicates" may represent the altered component of Mars earliest crust, which was churned by repeated impacts that were a common feature of the early solar system. That the bulk rock and breccia blocks within are altered to smectite clays suggests liquid water was probably stable that time and, at least in some locations, hydrothermal processes were occurring.
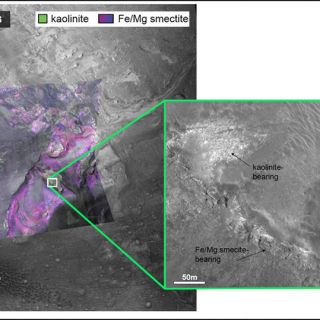
'Layered phyllosilicates': Near the two most areally extensive phyllosilicate deposits on Mars, at Mawrth Vallis and the Nili Fossae, a distinctive stratigraphy is observed, traceable over hundreds to thousands of kilometers. Alumnium phyllosilicates are found overlying Fe/Mg smectites. Figure 7 shows one example from eroded sediments which fill a crater. The bulk of the sediments are Fe/Mg smectite bearing, but a distinctive bright layer tens of meters thick instead has a spectral signature consistent with a kaolin group mineral most likely kaolinite or halloysite. Elsewhere on Mars, in this Al phyllosilicate layer, kaolinite is accompanied by montmorillonite and opaline silica (Bishop et al., 2008). A simple explanation to explain this stratigraphy is top-down leaching by surface and near surface waters that strips a pre-existing smectite unit of Fe, Mg, and Ca cations, leaving behind mostly Al and Si to form kaolinite (Ehlmann et al., 2009). An analog terrestrial setting is pedogenesis of Hawaiian basalts under intermittently wet conditions.
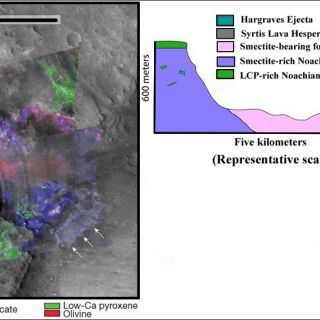
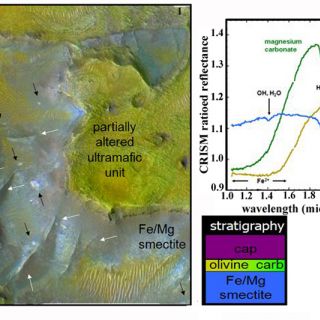
Carbonate and altered ultramafics: in one region of the planet, near the Nili Fossae an areally extensive stratigraphic unit elevated in olivine abundance (>30%) is found circumferential to the Isidis impact basin (Figure 8). The unit is thought to represent either lavas emplaced immediately following basin formation or a melt sheet containing materials excavated from Mars' lower crust and upper mantle by the impact (Mustard et al., 2009). The olivine-bearing bedrock exhibits signs of partial alteration, in most cases to magnesium carbonate and in a few cases to serpentine (Ehlmann et al., 2009). On Mars, these deposits are significant because the existence of carbonate at this site indicates neutral to alkaline aqueous conditions, in contrast to acid conditions indicated at different locations and in younger rocks by the MER rovers (Ehlmann et al., 2008b). Serpentine is also a tantalizing find because the process of serpentinization leads to the generation of methane. Although these particular rocks formed billions of years ago and do not indicate the source for the methane presently observed in the Mars atmosphere, it points to the fact that processes of methane production from serpentinization did exist in Mars' past.
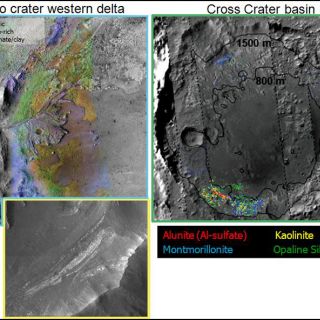
Fluvial/lacustrine sedimentary clays: Craters are topographic lows. A few have hosted crater lakes and still fewer host sedimentary structures that contain alteration minerals. Depending on the basin, however, the preserved sediments differ in mineralogy and implied geochemical conditions. One example is Jezero crater (Figure 9), a putative open basin lake which apparently hosted neutral to alkaline waters. The materials comprising sediments within the crater are Fe/Mg smectite- and Mg carbonate- bearing similar to the materials found in the Jezero basin watershed (Ehlmann et al., 2008a). Elsewhere, the lakes have a distinctly different acidic character. For example, a closed basin in Terra Sirenum, Cross Crater, probably once hosted a crater lake and is filled with sediments containing alunite, an Al-sulfate that forms only at pH <4 (Swayze et al., 2008). Alunite, along with silica and aluminum phyllosilicates, is found within friable layered units ringing the crater (Figure 9).
The sulfate-forming epoch and its many environments: Salts, e.g. sulfates, become increasingly common and clays less common in later time periods. As revealed by in-situ exploration by the Mars exploration rover at Meridiani Planum, the Hesperian sandstones there have been altered by acidic waters in a complex sequence of surface weathering and diagenesis (Squyres et al., 2004). At Gusev crater, the Spirit rover has found evidence for silica and sulfates possibly created by acid hydrothermal systems (Squyres et al., 2008). Furthermore, extensive silica deposits accompanied by sulfates have been found from orbit in the plains surrounding Valles Marineris and massive mounds of sulfate-bearing materials exist within the Valles system (Milliken et all, 2008; Murchie et al., 2009).
The future
All these discoveries point to the fact that Mars has a rich record from its first billion years available for exploration. On Mars, the new news for scientists is to think locally rather than globally. The types of chemical environments that hosted liquid water varied substantially in character through space and time.
The most immediate impact that these new mineral finds will have on NASA and ESA's program of Mars exploration is in the selection of the landing site for the next rover, the Mars Science Laboratory (MSL) that launches in 2011. Environments of paleolakes with sediments with Fe/Mg smectite clays (Holden and Eberswalde, Gale) and layered phyllosilicates (Mawrth Vallis) are currently represented in the suite of four finalists. In light of recent discoveries of the diversity of alteration environments on early Mars, two additional sites , altered ultramafics (Northeast Syrtis) and a plain of sediments of Fe/Mg smectites and chloride (East Margaritifer), were recently targeted for a period of intensive study for their consideration as candidates [for more on this selection process see http://marsoweb.nas.nasa.gov]. Landing site safety is the primary factor that must be fulfilled at the chosen site and efforts are ongoing to characterize any possible hazards. In addition to finding out more about aqueous processes on early Mars, scientists hope that in-situ sample analysis could reveal evidence for organic carbon (Mahaffy, 2009). Scientifically, an intense debate rages over (a) what types of environments early microbial organisms might have been most likely to inhabit and (b) in which types of environments biosignatures of past life-organic carbon, morphologic fossils, mineral disequilibria, or distinctive isotopic fractions-are most likely to be preserved over three billion years.
In spite of all the progress made since 2004, many questions persist about the newly discovered alteration minerals. One basic question is "how much?" Quantification of mineral abundance with VNIR data is a highly nonlinear problem, since texture and grain size also effect spectra, although efforts are beginning to model likely modal mineralogies (Poulet et al., 2008). Mineralogic data have also thrown down the gauntlet for geochemical modelers to use data on the minerals and their assemblages to more tightly constrain geochemical conditions and the rates and processes of mineral formation so as to establish more detailed parameters on Mars' earliest environments.
As VNIR images from OMEGA and CRISM continue to be collected and analyzed, discoveries of still more minerals on Mars may yet be made. By combining orbital data with data from the next generation of landers and rovers, we can make substantial progress in understanding the history of water on Mars, its evolution over billions of years, and perhaps, whether Mars ever hosted the geochemical environments necessary to sustain life.
References
Bibring, J.-P., et al. (2005), Surface diversity as revealed by the OMEGA/Mars Express observations, Science, 307, 1576- 1581.
Bibring J.-P., et al. (2006), Global mineralogical and aqueous Mars history derived from OMEGA/Mars Express data, Science, 312, 400-404.
Bibring, J.-P., et al. (2007), Coupled ferric oxides and sulfates on the Martian surface, Science, 317, 1206 - 1210.
Bishop, J. L., et al. (2008), Phyllosilicate diversity and past aqueous activity revealed at Mawrth Vallis, Mars, Science, 321, 830 - 833.
Burns, R.G. (1993) Mineralogic applications of Crystal Field Theory Cambridge Univ. Press, 527p.
Christensen, P.R. et al. (2000), Detection of crystalline hematite mineralization on Mars by the Thermal Emission Spectrometer : Evidence for near-surface water, J. Geophys. Res., 105, 9,623-9,642.
Clark, R. N., et al. (1990), High spectral resolution reflectance spectroscopy of minerals, J. Geophys. Res., 95, 12,653-12,680.
Clark, R. N., et al. (2003), Imaging spectroscopy: Earth and planetary remote sensing with the USGS Tetracorder and expert systems, J. Geophys. Res., 108(E12), 5131, doi:10.1029/2002JE001847.
Clark, R. N., et al. (2007), USGS Digital Spectral Library splib06a, U.S. Geol. Surv. Data, 231.
Clark, R. N., et al. (2008), Diversity of mineralogy and occurrences of phyllosilicates on Mars, Eos Trans. AGU, 89(53), Fall Meet. Suppl., Abstract P43D-04.
Ehlmann, B. L., et al. (2008a), Clay minerals in delta deposits and organic preservation potential on Mars, Nat. Geosci., 1, 355- 358.
Ehlmann, B. L., et al. (2008b), Orbital identification of carbonate-bearing rocks on Mars, Science, 322, 1828- 1832.
Ehlmann, B. L., et al. (2009), Identification of hydrated silicate minerals on Mars using MRO-CRISM: Geologic context near Nili Fossae and implications for aqueous alteration, J. Geophys. Res., 114, E00D08, doi:10.1029/2009JE003339.
Farrand W.H. et al. (2009) Discovery of jarosite within the Mawrth Vallis region of Mars. Icarus, 204, 478-488.
Gendrin A., et al. (2005), Sulfates in Martian layered terrains: The OMEGA/Mars Express View, Science, 307, 1587- 1591
Hartmann, W. K., G. Neukum (2001), Cratering chronology and the evolution of Mars, Space Sci. Rev., 96, 165-194.
Langevin, Y., et al. (2005), Sulfates in the north polar region of Mars detected by OMEGA/Mars Express, Science, 307, 1584-1587.
Mahaffy, P. (2009) Sample Analysis at Mars <http://www.geochemsoc.org/; : Developing Analytical Tools to Search for a Habitable Environment on the Red Planet", Geochemical News, 141.
Murchie, S. L., et al. (2007), Compact Reconnaissance Imaging Spectrometer for Mars (CRISM) on Mars Reconnaissance Orbiter (MRO), J. Geophys. Res., 112, E05S03, doi:10.1029/2006JE002682.
Murchie, S. L., et al. (2009), A synthesis of Martian aqueous mineralogy after one Mars year of observations from the Mars Reconnaissance Orbiter, J. Geophys. Res., 114, E00D06, doi:10.1029/2009JE003342.
Milliken, R.E. et al. (2008), Opaline silica in young deposits on Mars, Geology, 36, 847-850
Mustard, J. F., et al. (2008), Hydrated silicate minerals on Mars observed by the CRISM instrument on MRO, Nature, 454, 305- 309.
Mustard, J. F., et al. (2009), Composition, Morphology, and Stratigraphy of Noachian Crust around the Isidis basin, J. Geophys. Res., 114, doi:10.1029/2009JE003349.
Pelkey, S. M., et al. (2007), CRISM multispectral summary products: Parameterizing mineral diversity on Mars from reflectance, J. Geophys. Res., 112, E08S14, doi:10.1029/2006JE002831.
Poulet, F., et al. (2005), Phyllosilicates on Mars and implications for early Martian climate, Nature, 438, 623- 627.
Poulet, F., et al. (2008), Abundance of minerals in the phyllosilicate-rich units on Mars, Astron. Astrophys., 487, L41 -L44, doi:10.1051/0004-6361:200810150.
Roach, L. H., et al. (2009), Testing evidence of recent hydration state change in sulfates on Mars, J. Geophys. Res., 114, E00D02, doi:10.1029/2008JE003245.
Squyres, S. W., et al. (2004), In-situ evidence for an ancient aqueous environment at Meridiani Planum, Science, 306, 1709-1714.
Squyres S.W., et al. (2008), Detection of silica-rich deposits on Mars: Science, 320, 1063- 1067.
Swayze G.A. et al. (2008), Eos Trans. AGU, 89(53), Fall Meet. Suppl., Abstract P44A-04
Figure Captions
FIGURE 1. Topographic map showing large outflow channels and valleys draining into Mars' northern lowlands. In spite of abundant morphologic evidence for liquid water, until recently orbiting spectrometers, no large deposits of altered minerals had been found.
FIGURE 2. Chemical environments on Mars, adapted from Murchie et al., 2009 with mineralogic epochs from Bibring et al., 2006. The age dates for the Mars epoch boundaries are from Hartmann & Neukum (2001) cratering chronology.
FIGURE 3. Selected hydrated silicate minerals discovered by CRISM (Mustard et al., 2008; Bishop et al., 2008; Ehlmann et al., 2009). Spectra have been colored so as to match CRISM spectral data to the most probable mineralogic match, based on examination of laboratory spectra. In some cases, mineral identifications cannot be made uniquely (e.g. muscovite vs. illite, orange/yellow) or phases on Mars are intermediate between endmember minerals with solid solution series (e.g. Fe/Mg smectite with composition between nontronite and saponite endmembers, blue/cyan)
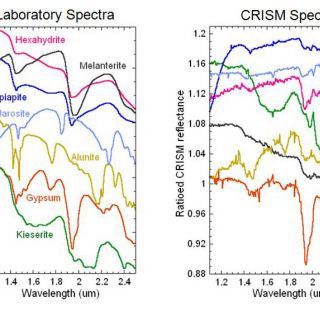
FIGURE 4. Selected sulfate minerals discovered by CRISM (Swayze et al., 2008; Murchie et al., 2009; Roach et al., 2009)
FIGURE 5. ‘Deep phyllosilicates' as exposed in a 600m tall scarp beneath a mafic LCP-bearing cap rock (figures after Mustard et al., 2008; 2009). Arrows indicate that Fe/Mg smectites exposed beneath the younger Syrtis Major lavas. Olivine is present here in as mobile sands. The two CRISM images are overlain on and use to colorize a grayscale, high resolution Context Imager (CTX) image. Uncolored parts of the image do not have CRISM coverage.
FIGURE 6. ‘Deep phyllosilicates' as excavated by impact. Mineral mapping from several CRISM images shows that prehnite and chlorite are the spectrally dominant phases in ejecta surrounding this 50km diameter crater (figure from Ehlmann et al., 2009). Where CRISM images are black, no alteration mineral phases were observed during examination of spectra.
FIGURE 7. Example of the layered phyllosilicates. A CRISM parameter map was used to colorize a higher resolution Context Imager (CTX) observation. The 2.3 micron Fe- or Mg-OH absorption in Fe/Mg smectites is mapped as red, the H2O 1.0 micron band is mapped as blue, and the 2.2 micron Al-OH band is mapped as green. In this filled crater from Nili Fossae, a thin Al-OH bearing unit, identified using spectral data as kaolinite, overlies an Fe/Mg smectitie-bearing unit (figure from Ehlmann et al., 2009).
FIGURE 8. Example of the partially altered ultramafic unit and phyllosilicate/carbonate stratigraphy. CRISM spectra at the upper right correspond to colored units in this CRISM false color plus grayscale HiRISE (23cm/pixel) composite image. Arrows point to raised fractures (white) and breccia blocks (black) in the Fe/Mg smectite-bearing unit (figure from Ehlmann et al., 2009).
FIGURE 9. (left) the western delate of Jezero crater. Sediments within are Fe/Mg smectite and Mg carbonate bearing. The inset shows the wall of the crater in the delta which exposes a 20m crossbedding structure (Ehlmann et al., 2008a). (right) the Cross crater basin hosts layered sediments and where eroded exhibit signatures of alunite, silica, and Al-phyllosilicates (Swayze et al., 2008).