By Katharina Loddersa
aDept. of Earth & Planetary Sciences and McDonnell Center for the Space Sciences, Washington University, Saint Louis MO 63130
Received February 2010; accepted in revised form 18 March 2010; available online 23 March 2010
Synopsis
This is a brief description of historical and more recent studies about the composition and chemistry of the giant planet atmospheres, with an emphasis on Jupiter. There are several constraints from the chemistry for the various models of gas-giant planet formation, but our current knowledge remains incomplete, despite the tremendous progress made during the past 100 years of planetary research and technical developments. This article is intended to stimulate some interest in research about the gas-giant planets rather than being a comprehensive review.
Some General Characteristics
The gas-giant planets Jupiter, Saturn, Uranus and Neptune in our solar system are quite different in mass, density, and in chemical composition than the inner terrestrial planets Mercury, Venus, Earth, and Mars. Several physical properties of the gas giant planets and their atmospheres are in Table 1 which illustrates the close kinship of Jupiter and Saturn as well as that of Uranus and Neptune. Jupiter and Saturn, the most massive planets in the solar system are mainly composed of H and He, whereas in the Earth, oxygen is the most abundant element, mainly tied to rock, but also abundant in the oceans and air. The elemental make-up of the giant planets especially that of Jupiter and Saturn, is much closer to the solar photospheric composition than to the terrestrial planets. However, unlike the solar photosphere with relatively 'simple' chemistry of neutral and ionized atoms, the cool outer atmospheres of the giant planets are rich in molecular chemistry.
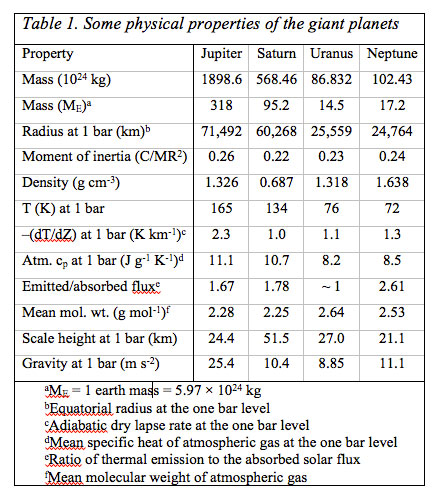 |
The giant planets fall into two groups: The larger and gas-rich giants Jupiter and Saturn with more than 50 mass % H and He, and the smaller gas-poor giants Uranus and Neptune with less than 50 mass % H and He. Jupiter's low bulk density (1.33 g cm-3) and Saturn's even lower density (0.69 g cm-3) coupled with their atmospheric composition, size, shape (i.e., their oblateness), and interior structure models are evidence that they are mainly H and He with smaller amounts of heavier elements. Uranus and Neptune have higher densities, and presumably larger contents of compounds containing C, N, and O.
The oxidizing atmospheres of Venus, Earth, and Mars are < 0.01% of the planets' total masses and are terminated by sharp boundaries with their surfaces. In contrast, the reducing atmospheres of the giant planets are significant fractions of the total planetary masses. There are no observable solid surfaces, and (apparently) bottomless atmospheres extend deep into the planets. In absence of something similar to sea-level as on Earth or a mean radius of the solid surface as on the other terrestrial planets to which atmospheric properties are referenced, the reference used is the one bar level in the atmospheres of the giant planets. Their atmospheres extend to great depth, but may turn into liquid metallic H-He deep inside Jupiter and Saturn, while ionic oceans of aqueous ammonia may occur deep inside Uranus and Neptune. All four planets have magnetic fields, which are probably generated by dynamo currents in the electrically conductive fluids (liquid metallic H-He inside Jupiter and Saturn and ionic oceans of aqueous ammonia inside Uranus and Neptune). At least three of the four giant planets emit more energy than they receive from the Sun (Uranus is the apparent exception). The heat emitted by the giant planets comes from their continued gravitational contraction and cooling and also from phase separation and sedimentation of He from H in their deep interiors (on Saturn and Jupiter). In contrast, the terrestrial planets are in radiative equilibrium and emit only as much energy as they absorb from the Sun.
Theoretical models suggest that the observed heat fluxes on Jupiter, Saturn, and Neptune are transported by atmospheric convection. This has been demonstrated for Jupiter by in situ measurements down to 22 bars from the Galileo probe (Seiff et al. 1998) and for Saturn, Uranus, and Neptune by determination of P, T profiles down to ~ 10 bars from Voyager IRIS data (Lindal 1992, Lindal et al. 1992). Convection is also necessary to explain observations of CO, PH3, AsH3, and GeH4 at abundances orders of magnitude larger than their thermochemical equilibrium values in the upper troposphere (~ 100mb to ~ 3 bar) where the spectral absorption bands form and the identity of the molecules is revealed though vibrational-rotational transitions.
Discovery Log About Giant Planet Chemistry
It was in the early 1900s when the first spectroscopic investigations were undertaken to find the chemical nature of the gas-giant planets Jupiter Saturn, Uranus, and Neptune. The discovery of the latter two planets itself was not too long ago: Uranus in 1781, and Neptune in 1846 (The dwarf planet Pluto, an icy world which we do not discuss here, was only discovered in 1930).
Obtaining the spectral fingerprints of the chemistry in their atmospheres was not a trivial task. At the time observers had to be chemists as well as astronomers in order to develop emulsions to increase the sensitivity of photographic plates that could capture the planetary spectra they sought. The chemistry did not stop there, however. In 1909, Slipher obtained spectra of the giant planets, only to realize that the identification of the chemical species causing the numerous absorption bands would require further detective work. Slipher noted 'Of the chemical identity of the spectral bands of these four planets almost nothing is certainly known.' There was a hint of atomic hydrogen absorption, but no firm conclusions were possible. He compared several of the unknown bands to bands at wavelengths that appear in cool stars but he ruled out any similarities to gases there, and others after Slipher came to the same conclusions. Slipher discussed the possible water absorption in Uranus and Neptune but also showed that these water bands are not due to absorption in these planets but instead stem from telluric water vapor instead. Many other bands in the spectra remained a mystery. Slipher concluded: 'The remaining bands in these spectra are yet to be chemically accounted for. The spectra are quite unlike the spectrum of the Earth's atmosphere, but the differences of pressure and temperature between the atmosphere of the major planets and our air are probably large. Until the bands are identified, it is not possible to arrive at satisfactory conclusions of the conditions existing in the atmospheres, although the breadth and diffuseness of the bands suggest high pressure or high temperature or both.'
By the 1930s, knowledge about the physical conditions and chemical composition of the giant planets was improving. In 1930, Menzel described the two possibilities for the physical state of the giant planets that were popular at the time: 'Either their interiors are hot and gaseous, i.e., greatly distended by high temperature, or the material they are composed of is of relatively low density.' Up to that time, the first possibility had been widely entertained, but the discovery that the surface temperatures of Jupiter and Saturn were only about 150K, a little higher than expected from radiative balance with solar radiation (Menzel 1923), eventually ruled against the 'hot' giant planet hypothesis. Modern measurements yield temperatures from 165 k( Jupiter) to 72 K (Neptune) at the 1 bar level; see Table 1.
The low observed mean densities and estimates for the mass distribution within the planets derived from the moment of inertia led to the suggestion that the giant planets consist of a central solid core made of rock and metal, surrounded by a layer of ice upon which a large atmosphere is located. The first of such layered structure models was by Jeffreys (1923, 1924) but at that time it was not yet known that the atmospheres are mainly H-He rich followed by smaller amounts of methane and ammonia. Layered structure models, although modified and much refined, of the giant planets are widely accepted today. Often the cores of the giant planets are still referred to as rock and ice. 'Rocky' elements are those usually found in metal, silicate, and sulfide rock (e.g., Ca, Al, Fe, Mg, Si, S) and 'icy' elements (e.g., Ar, C, N, O) are those expected to condense as various ices in the solar nebula. However, whether distinct cores actually exist or all of the 'rocky' and 'icy' elements are dissolved in the molecular and/or metallic H - He phases is unclear at present. At least some of the 'rocky' and 'icy' elements are present in the molecular H - He layers of Jupiter and Saturn because CH4, NH3, H2O, H2S, AsH3 (arsine), GeH4 (germane), and PH3 (phosphine) are observed in their atmospheres. The Juno polar orbiter, scheduled for launch in August 2011, will measure Jupiter's gravitational field with sufficient accuracy to decide whether or not a core exists.
The low densities of the gas giants require that the outer portions are made of compounds of low molecular weight. Jeffreys considered the low-density liquids and solids hydrogen, helium, nitrogen, oxygen, CO, CO2, CH4, and C2H6. A large gaseous portion was clearly required to account for Saturn's density below that of water. Based on material properties, Jeffeys (1924) suggested that the atmospheres of Jupiter and Saturn probably consist of hydrogen, nitrogen, oxygen, helium, and perhaps methane, and make 9% and 23% of the entire radius of Jupiter and Saturn, respectively. Except for O2, this idea about which gases are possible in the outer atmospheres is correct. However, the abundances needed to be determined, and other gases, namely He, needed to be added. He also thought that there are clouds of solid CO2, which is not the case. Still, some types of clouds were necessary since observations indicated that Jupiter (and the other giant planets) do not rotate as rigid spheres but have visible surface features that change.
Menzel (1930) concluded that the outer atmospheres are mainly hydrogen based on the recognition from the previous year, that the sun, like other stars, is mainly composed of hydrogen (Russell 1929). This is in accord with the earlier tentative observation of hydrogen by Slipher. However, the collision induced absorption of H2 was only found decades later in the 1950s (e.g., see review by Rea 1962).
However, identification of the gases in the atmospheres of the giant planets still remained to be done. What compounds caused the strong absorption bands in the giant planet spectra that Slipher and others had observed? It was quite clear that some strong absorbers turned Uranus and Neptune pale cyan-greenish through absorption at red visible wavelengths. Later it was found that absorptions were strong in the infrared as well - a feature now exploited to hunt and identify possible gas-giant planets around other stars. The culprits causing the strong absorptions in the giant planets - methane and ammonia - were identified by Wildt in 1932. These spectral identifications became more firm later, as more laboratory measurements became available.
The identification of hydride-gases CH4 and NH3 made sense as well from the theoretical point of view. They are hydrides of the more abundant elements C and N, and they are stabile at low temperatures. One would also expect the hydride of oxygen, H2O. However, while detection of water vapor is technically possible, it was not observed (until the 1980s) because all water vapor is frozen out into clouds in the deeper, unobservable regions of the atmospheres of the gas giant planets. The water vapor pressure above the water clouds is very low at the temperatures where observations probe the atmospheres. Indeed, the water abundance on the giant planets still remains an open question (see below).
By the early 1960s, many methane and ammonia absorption features were identified in giant planet spectra (e.g., see review by Rea 1962). Estimates of abundances were made, but these were quite uncertain. Molecular H2 was first detected in Uranus and Neptune, but then also found in Jupiter and Saturn. The presence of helium on Uranus was inferred on theoretical grounds to match the planet's density with the deduced H2 abundances. Also identified by that time were ammonia clouds on Jupiter. However, no other compounds had been identified in the gas giant planets up to the early 1960s.
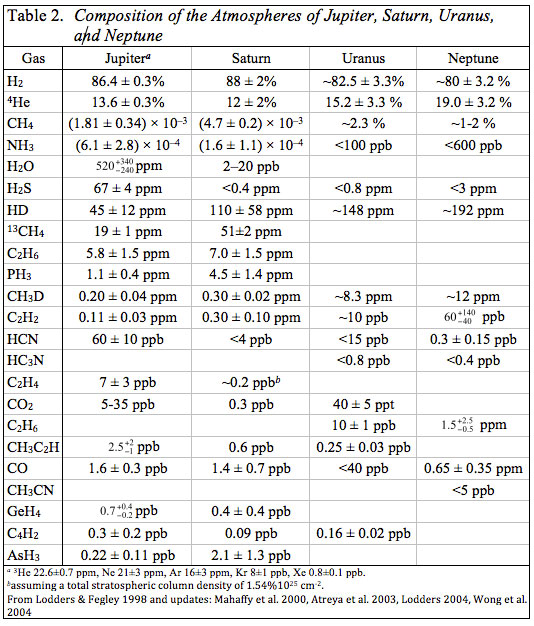
This does not mean that searches for other molecules were lacking. Volatile hydrides of elements such as Si, Ge, P, and As are among the gases that could be present on thermochemical grounds. The abundances of these gases can be used to constrain mixing processes, and therefore place constraints on the interior of the outer planet atmospheres. Detection and quantitative determinations of deuterated molecules such as HD and CH3D could provide information about the proto-solar D/H ratio (also of interest to cosmologists) because D is destroyed in the sun. Furthermore, the increasing D/H ratios in the giant planets with increasing distance from the sun could indicate ion-molecule chemistry in the outer solar nebula or the efficiency with which cold interstellar materials were homogenized in the outer solar system (see Fegley 1999 for a review). The development of high resolution Fourier Transform Infrared (FTIR) spectrometers led to the discoveries of many other gases from the late 1960s onwards. Table 2 gives the composition of the atmospheres of the gas giant planets compiled from literature data.
 |
As most information is for the atmospheres of Jupiter (and Saturn), we focus on the largest planet in the solar system. Jupiter has a mass (MJ) of about 10-3 that of the Sun, or approximately 318 times that of the Earth (ME). Saturn is the second most massive gas giant planet with a mass of about 95 ME. Although quite massive relative to the Earth and other planets in our solar system, Jupiter is much smaller than an object capable of 'burning' deuterium by nuclear fusion which requires at least 13 times Jupiter's mass. The 13 MJ mass limit is widely taken as the boundary between planets (at lower masses) and brown dwarfs (at higher masses).
The molecular H - He layers of Jupiter and Saturn are only a small fraction of each planet. However, these layers are the only regions of the two planets that are observable from the Earth and spacecraft. Our knowledge of atmospheric composition and structure on Jupiter and Saturn is based on a combination of Earth-based and spacecraft observations. The spacecraft which explored Jupiter and Saturn are Pioneer 10, Pioneer 11, Voyager 1 and 2, Galileo (7 Dec 1995 arrival at Jupiter and entry probe launch - 21 Sept 2003), and Cassini (1 July 2004 arrival at Saturn, and continuing now).
In situ observations of Jupiter's atmosphere by the Galileo entry probe (7 December 1995) extended down to ~ 22 bars (at 429 K), about 126 km below the one bar level. Saturn's atmosphere has not yet been explored by entry probes. (The Huygens probe on the Cassini spacecraft descended to the surface of Titan, Saturn's largest satellite, on 14 January 2005.) The New Horizons mission launched in 2007 had a Jupiter flyby en route to the dwarf planet Pluto and its three satellites Charon, Hydra, and Nix.
In general remote sensing observations in the UV, visible, and IR regions sample only the uppermost regions of Jupiter's and Saturn's atmospheres down to pressures of a few bars. Radio wave observations extend deeper to tens or hundreds of bars.
Visually, Jupiter's atmosphere shows dark belts alternating with bright zones. These are the upward (bright zones) and downward (dark belts) arms of atmospheric convection cells. The Great Red Spot (about 32,000 km long and 13,000 km wide) in Jupiter's southern hemisphere exists since at least 1664 when it (or a similar feature) was observed by the English scientist Robert Hooke (1635 - 1703). The Great Red Spot is a long-lived cyclonic storm and its red color is likely due to red phosphorus formed by UV sunlight photolysis of PH3 gas. Other ovals and spots have also been observed in Jupiter's meteorologically active atmosphere for various periods of time. Inorganic compounds of sulfur and other elements probably are the chromophores coloring the belts in Jupiter's atmosphere. The organic compounds produced by atmospheric photochemistry are colorless and do not provide the observed cloud colors. The bright zones are high level NH3 ice clouds at about 0.5 bars (138 K). Saturn's atmosphere is less colorful than Jupiter's but has a similar banded structure visible in spacecraft photographs.
Remote sensing observations by the Voyager spacecraft and in situ measurements by the Galileo entry probe show that Jupiter's atmosphere is convective down to at least 22 bars where the temperature is 429 K. The presence of CO, AsH3, GeH4, and PH3 at abundances orders of magnitude greater than their thermochemical equilibrium values in the upper tropospheres of Jupiter and Saturn shows that convective mixing extends down to at least kilobar levels in their atmospheres. The convective regions (tropospheres) of Jupiter's and Saturn's atmospheres extend up to their tropopauses, which are the radiative - convective boundaries and temperature minima in their atmospheres (~100 mb, 110 K on Jupiter and ~83 mb, 83 K on Saturn). Methane in the stratospheres of the giant planets plays the role that O3 does in Earth's stratosphere and absorption of UV sunlight by CH4 heats the stratospheres of the four gas giant planets.
Chemical Processes
Chemistry in the atmospheres of the gas giants is determined by their overall elemental composition, and the stability of chemical compounds at the prevailing temperatures, the (gravity-controlled) total pressures, and where applicable in the atmosphere, the interaction with UV light and charged particles. The following describes some characteristic chemical processes.
Both photochemistry and thermochemistry control atmospheric chemistry on the giant planets. Ultraviolet sunlight and other disequilibrating energy sources such as charged particles (e.g., in Jupiter's magnetosphere) and cosmic rays drive photochemical reactions in the upper atmospheres of all four planets. The heat released by gravitational contraction and cooling (and by He phase separation and sedimentation on Jupiter and Saturn) drives thermochemical reactions in the deep atmospheres. Thermochemical reactions also lead to formation of condensation clouds throughout the observable and deeper, unobservable regions of all four giant planets. There are intermediate regions where both photochemistry and thermochemistry affect the chemistry in the giant planet atmospheres, and the depth of these intermediate regions varies from planet to planet.
Ultraviolet sunlight drives photochemistry in the upper atmospheres of the four giant planets Jupiter, Saturn, Uranus, and Neptune. In turn, photochemistry moves the upper atmospheres of these planets away from equilibrium and produces disequilibrium species (e.g., ethane C2H6, acetylene C2H2, and ethylene C2H4) from methane and hydrazine N2H4 from ammonia, the thermodynamically stable forms of carbon and nitrogen in the H2-rich atmospheres of the giant planets. In addition, photochemistry of NH4SH and phosphorus compounds is plausibly responsible for the colored belts and bands on Jupiter. The first modern models of hydrocarbon and ammonia photochemistry on Jupiter emerged in the late 1960s.
Many of the gases observed in their atmospheres are hydrides, e.g., CH4, NH3, H2O, H2S, PH3, GeH4, and AsH3. All of these gases (except H2O and H2S) are photochemically destroyed by UV sunlight in the stratospheres of Jupiter and Saturn. Ammonia is removed by formation of NH3 ice clouds and photolysis above these clouds in the atmospheres of Jupiter and Saturn. Water vapor and H2S condense to form clouds (liquid water, NH4SH) before they reach the stratospheres where they can be photolyzed by UV sunlight. As shown in Table 2, NH3 is more abundant than H2S on Jupiter and NH4SH condensation removes all H2S, but not all NH3 from Jupiter's atmosphere above the NH4SH cloud. As long as nitrogen and sulfur are equally enriched (or depleted) relative to solar composition this is probably also true on the other giant planets because the atomic N/S ratio is 5.0 in solar composition material.
Hydride gases are important in the atmospheres of all four giant planets because H2 is the major gas and high atmospheric pressures exist in their hot, deep atmospheres where thermochemical reactions proceed rapidly. Thus, any gases or solids produced by photochemistry in the upper atmospheres of Jupiter and Saturn are rapidly converted back into hydrides by thermochemical reactions deep inside these planets. For example, CH4 photochemistry forms ethane C2H6, which has an abundance of 5.8 - 7 parts per million by volume on Jupiter and Saturn. At ~ 1000 K deep inside these two planets it reacts with H2 to reform CH4 via the net reaction C2H6 + H2 = 2CH4. Other photochemically produced species (e.g., C2H2, C2H4, CH3C2H, C4H2, N2, elemental As, Ge, and P) that are transported downward into the hot, high pressure regions of the atmospheres of Jupiter and Saturn also react with H2 to reform hydrides.
The presence of hydride gases is important for several other reasons. Hydrogen sulfide was first observed and measured in Jupiter's atmosphere by the Galileo Probe Mass Spectrometer (GPMS). The observed H2S/H2 ratio of ~ 7.8 × 10-5 is about 2.7 times higher than the S/H2 ratio of ~ 2.9 × 10-5 based on solar elemental abundances. (The S/H2 ratio and other element/H2 ratios based on solar abundances are calculated using ½ the H elemental abundance, which gives the equivalent H2 abundance in solar material.) The presence of H2S in Jupiter's atmosphere requires depletion of Fe by Fe cloud condensation at high temperatures deep in Jupiter's atmosphere. If Fe cloud formation did not occur, H2S would be completely absent from Jupiter's atmosphere because FeS (troilite) formation at ~ 700 K would consume all H2S gas (because the atomic ratio of Fe/S ~ 2 in solar composition material) via
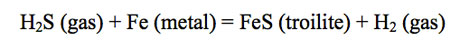 | (1) |
Hence, Fe metal condensation clouds form at great depth inside Jupiter according to thermochemical equilibrium models.
Similar thermochemical equilibrium models predict H2S is the dominant sulfur gas in the atmospheres of Saturn, Uranus, and Neptune. Hydrogen sulfide is not observed by remote sensing on these planets because formation of NH4SH clouds (via reaction of H2S with NH3) depletes H2S from the observable regions of their atmospheres. However, observation of H2S by the Galileo entry probe shows that planetary entry probes should be able to detect H2S on Saturn, Uranus, and Neptune.
The presence of CH4 and GeH4 (germane), but not of SiH4 (silane) on Jupiter and Saturn is due to condensation of magnesium silicates (MgSiO3, Mg2SiO4) deep in their atmospheres. Silicon is much more abundant than germanium in solar composition material (the atomic Si/Ge ratio is ~ 8,700), but it is more refractory than Ge and forms silicate clouds deep in the atmospheres of the giant planets via net thermochemical reactions such as
| (2) |
and
| (3) |
Silane is not observed on either Jupiter or Saturn and the observational upper limits for it are about one part per billion by volume (SiH4/H2 ratio < 10-9) on both planets). This is 68,000 times smaller than the Si/H2 ratio of 6.8 × 10-5 from solar elemental abundances. In contrast, the observed CH4/H2 ratios of 2.1 × 10-3 on Jupiter and 5.3 × 10-3 on Saturn are 4 to 11 times higher than the C/H2 ratio of 4.9 × 10-4 from solar elemental abundances. The GeH4/H2 ratios of 8.1 × 10-10 (Jupiter) and 4.6 × 10-10 (Saturn) are 0.1 to 0.06 times the Ge/H2 ratio of 78 × 10-10 from solar abundances. The Ge/H2 ratios in the atmospheres of Jupiter and Saturn are lower than the solar value because not all Ge in their atmospheres is present as germane. Most Ge condenses out of the atmospheres of Jupiter and Saturn as elemental Ge and Ge chalcogenides at high temperatures.
Aside from photochemically produced gases, the hydride/H2 ratios in the atmospheres of Jupiter and Saturn give elemental enrichments (or depletions) relative to solar composition. As already seen, carbon and sulfur are enriched and germanium is depleted relative to solar composition in Jupiter's observable atmosphere. Carbon is also enriched on Saturn (~ 11 times solar) but H2S is not observed as discussed earlier. Nitrogen is enriched 4.9 times solar (with 46% uncertainties) on Jupiter, but only 1.2 times solar on Saturn where condensation of NH3 into NH3 ice clouds depletes ammonia in the observable region of its atmosphere. Water is depleted and is only 0.6 times solar on Jupiter. This depletion is observed below the level where water clouds are predicted to form and is probably a depletion of water (and oxygen) throughout Jupiter's atmosphere and interior. On Saturn H2O is removed by condensation of water clouds below the observable region of Saturn's troposphere. The small amounts of H2O observed in Saturn's stratosphere are due to oxygen coming into Saturn's upper atmosphere from its icy rings, and provide no information on the water content of the planet.
Phosphorus is enriched 2.2 times solar on Jupiter and 9 times solar on Saturn. Arsenic is depleted on Jupiter (~ 0.6 times solar) and enriched on Saturn (~ 5.8 times solar). Arsine is the major As-bearing gas on Jupiter and Saturn, but condensation of elemental arsenic at 400 K depletes the AsH3 abundance in the cooler, observable region of Jupiter's atmosphere. Arsenic and phosphorus behave similarly in meteorites and in the solar nebula, so their enrichment factors on Jupiter and Saturn are plausibly the same. This is the case within the uncertainties on the enrichment factors for As and P on Saturn.
These data on hydride/H2 ratios show that the atmospheres of Jupiter and Saturn are close to solar composition and that Saturn is more enriched in heavy elements relative to solar composition than Jupiter. This trend is continued at least qualitatively by Uranus and Neptune where CH4/H2 ratios are about 57 and 38 times larger relative to solar composition, respectively. Given their mean densities, similar sizes, and uncertainties in the CH4 observations, it is plausible that Neptune is more enriched than Uranus. Except for photochemically produced gases and isotopomers (e.g., CH3D), no other hydride gases are observed on Uranus or Neptune. Thus the heavy element enrichment trend on the four giant planets depends on observations of methane.
Chemical reaction rates and vertical mixing rates also explain the abundances of CO, PH3, GeH4, and AsH3 in the atmospheres of Jupiter and Saturn. The abundances of these gases are significantly higher than those predicted at chemical equilibrium in the cool, observable regions of the atmospheres of Jupiter and Saturn. Carbon monoxide and PH3 are the most dramatic examples because their observed abundances are about 32 to 36 orders of magnitude greater than their chemical equilibrium values. The reason for this is rapid vertical transport of CO and PH3 from hot, high pressure atmospheric regions where they are more abundant due to favorable chemical equilibria.
For example, consider CO in Jupiter's atmosphere. Carbon monoxide is produced by oxidation of methane via
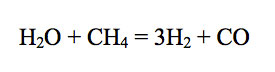 | (4) |
The equilibrium constant for reaction (4) in terms of partial pressures is
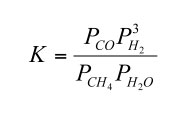 | (5) |
If we rewrite this to solve for the CO/CH4 ratio at equilibrium we obtain
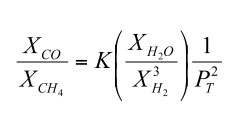 | (6) |
Thermodynamic data show that the equilibrium constant for reaction (4) increases with increasing temperature. Furthermore, the adiabatic gradient (dP/dT) in the convective lower atmosphere of Jupiter is easily calculated using the temperature dependent heat capacity of H2 - He gas mixtures. Thus, the CO/CH4 ratio as a function of temperature (and thus of depth and pressure) can be computed from the observed H2O and H2 abundances. These calculations show that the observed CO/CH4 ratio of ~ 8.8 × 10-7 occurs at about the 1100 K level in Jupiter's atmosphere.
Chemistry and Gas-giant Planet Formation Ideas
What is known about the gas-giant atmospheric composition and chemistry also provides clues to the formation mechanism. The non-solar elemental compositions show that chemical fractionations between the rock, icy, and gases (H2, noble gases; see below for more about water, Ar, Kr, Xe,) took place. As soon as the first hints of Jupiter's and Saturn's composition became available, the models came. With respect to planet formation, Menzel's line of thought in 1930 was that the giant planets formed at the same time as the sun from the same materials. Density differences among the planets could arise when light gases, especially hydrogen, were lost from giant planets early on, when the planet was extended through heating by release of gravitational energy. These thoughts about formation and modifications of planetary composition during planet formation and evolution now find renewed attention in the context of other giant planets discovered outside the solar system. Currently, two competing models portray how the gas giant planets could have formed from the materials in the accretion disk (see, e.g., Pollack et al. 1996, Boss 2001). The core accretion model postulates that such planets form after enough solid and icy materials accumulated to form a core of 5-10 Earth-masses which can gravitationally capture H and He rich gas before the planetary accretion disk is dissipated. This requires rapid accumulation of solids which may be difficult within the estimated lifetime of the solar nebula. Thus the amount of H and He captured depends on the accumulation rate of the 'core' and the rate of capture. The advantage of this model is that it can account for higher heavy element contents than expected for solar composition if capture of H and He is terminated before all gas in the accumulation region of the planet could be acquired by the planet.
The other formation model postulates that planets like Jupiter form through gravitational instabilities in the accretion disk, more similar to the mechanism the sun formed. This allows rapid planet formation. A consequence of this model is that the planet would be made of elements and compounds that are present in the accretion disk without any fractionation of rocky and icy from H and He. However, subsequent fractionation, such as loss of light gases, could also lead to an overall composition which is heavy element rich when compared to the sun.
Lessons From Water and Chemically 'Inert' Gases
The Galileo Probe Mass Spectrometer also observed Ne, Ar, Kr, and Xe in Jupiter's atmosphere (Table 2). The observed noble gas/H2 mixing ratios are 0.1 (Ne), 2.9 (Ar), 2.4 (Kr), and 2.5 (Xe) times relative to solar composition. Neon and Ge are depleted by the same factor (0.1) but for different reasons - Ne is thought to partition preferentially into helium in Jupiter's interior while most Ge condenses out of Jupiter's atmosphere as elemental Ge and Ge chalcogenides at high temperatures. In contrast, Ar, Kr, and Xe are about as enriched as S (2.7 times) relative to solar composition.
The relatively uniform enrichment of the heavy noble gases (Ar, Kr, Xe) on Jupiter requires that they were not fractionated, which limits the possible scenarios of how these gases were acquired by the planet. One possibility is that they were also captured from the solar nebula when the large amounts of H2 and He were captured. This, however, requires subsequent fractionation of H2 from the noble gases because the observed noble gas/H2 ratio is not solar, i.e., not the ratio that prevailed in the solar nebula. Such fractionation can come from subsequent atmospheric loss of H2, or removal of H2 into the liquid and metallic H-He layers deeper in the planet (see Lodders 2004 for a discussion).
Another way to explain the uniform noble gas enrichment is their delivery in icy planetesimals. The heavy noble gases, as well as CO and N2, can be captured into clathrate hydrate 'cage' compounds X·nH2O, where X is the caged atom or molecule, and n, with typical values of 5-7, is the number of water molecules providing the 'cage'. If clathrate hydrates form at sufficiently low temperatures, quantitative removal of the noble gases from solar nebula gas could be achieved, but He and H2 stay behind. If clathrate-bearing icy planetesimals were captured, a planet accreting such ices receives the same unfractionated noble gas abundances. Such a model is advocated by Gautier et al. (2001). Similarly, the noble gases may get trapped into amorphous ice, again at low temperatures (Owen et al. 1999). The problem is that quantitative noble gas clathration requires very low temperatures that can only achieved at larger distances from the sun than Jupiter's current location. Formation of crystalline clathrate hydrates may be less likely than amorphous ice formation, but in either case, both crystalline and/or amorphous ices have to quantitatively capture the noble gases and then move inward to be accreted by the planet. This may lead to selective release of light noble gases because the ices get heated as they get closer to the sun. Then the planet no longer receives ices with unfractionated noble gas abundances (see Lodders 2004, Guillot and Hueso 2006 for various arguments). Alternatively, larger water abundances in the Jupiter region might have increased clathration efficiency (Gautier and Hersant, 2005). Yet another model is to assume incorporation of noble gases into clathrates or amorphous ices, inward transport and sublimation of these ices deliver the noble gases unfractionated. Over time as the accretion disk evolves, an enrichment of the noble gases would result in the inner solar system as redistribution of ices from the outermost regions proceeds (Guillot and Hueso 2006). All these models make specific predictions about the heavy noble gas abundances on Saturn, Uranus, and Neptune, but without atmospheric entry probes measuring these abundances, all these models remain possibilities for the moment.
Another obstacle from accretion of clathrate hydrates or amorphous ice is that large amounts of water are brought to the planet. The expected water abundances are higher than the solar O/H2 ratio even if all solar O were present as water. The amount of O in the form of water in a solar composition gas available for forming clathrates is barely sufficient to provide 'cages' for the noble gases (see Lodders 2004). Since other gases such as N2 and CH4, as well as ammonia-hydrate, NH3 H2O, compete for water, the amount of water must be higher than given by the solar value. Enrichment of water ice through cold-trapping in the outer solar system is a very likely possibility, so supply of water for ice formation is not necessarily a problem. However, the ice that carries the noble gases to the planet atmosphere also brings water, thus, a water enrichment should be observed below the water-ice clouds that are present in Jupiter's upper atmosphere.
However, the available observations indicate that the O/H2 ratio on Jupiter is 0.6, clearly sub-solar. The Galileo probe measurements could be biased since the probe entered a 'hotspot' which is an uncharacteristically dry pocket in the atmosphere caused by meteorological phenomena. However, other observations also indicate a relatively dry atmosphere. If the noble gases where brought in water ice, and/or the planet formed by core accretion of a massive rocky and icy core (see below), water must be somewhere on Jupiter. At the minimum, one should expect a solar complement of oxygen, even if this where still at odds with the enrichment of other rocky and icy elements relative to solar.
The other option is that Jupiter never acquired large amounts of water, but instead, the role of water during planet formation was replaced by refractory organic 'goo' that aided the accretion of a substantial rocky-icy-organic core onto which solar gas was captured (see Lodders 2004 for a detailed description). The decision which formation scenario is 'right' can only be decided by future measurements of the deep water content of Jupiter, preferentially by emerging probes into more characteristic atmospheric regions, but also on the other giant planets. This would not only improve the constraints on gas-giant formation models, but also place constraints on the kind and distribution of planetary building blocks within the forming solar system.
References
Atreya, S.K, Mahaffy, P.R., Niemann, H.B., Wong, M.H., Owen, T.C. 2003, Composition and origin of the atmosphere of Jupiter - an update, and implications for the extrasolar giant planets, Planet. Sp. Sci. 51, 105-112
Boss, A.P. 2001, Gas Giant Protoplanet Formation: Disk Instability Models with Thermodynamics and Radiative Transfer Astrophys. J. 563, 367-373
Fegley, B. & Lodders, K. 1994, Chemical models of the deep atmospheres of Jupiter and Saturn. Icarus 110, 117-154
Fegley, B. 1999 Chemical and physical processing of presolar materials in the solar nebula and implications for preservation of presolar materials in comets. Space Sci. Rev. 90, 239-252
Gautier, D. Hersant, F., Mousis, O., Lunine, J.I. 2001, Enrichments in Volatiles in Jupiter: A New Interpretation of the Galileo Measurements, ApJ., 550, L227-
Guillot, T. Hueso, R., 2006, Jupiter's composition: Sign of a (relatively) late formation in a chemically evolved protosolar disk, Mont. Not. R. Astron. Soc. 367, L47-L51
Jeffreys, H. 1923, The constitution of the four outer planets, Month. Not. Roy. Astron. Soc. 83, 350-354
Jefferys, H. 1924, On the internal constitution of Jupiter and Saturn, 84, 534-538
Lewis, J.S. Prinn, R.G., 1984 'Planets and their atmospheres: Origin and evolution,' Academic Press, Orlando, FL, 1984
Lindal, G.F., Lyons, J.R., Sweetnam, D.N., Eshleman, V.R., Hinson, D.P. 1987, The atmosphere of Uranus - Results of radio occultation measurements with Voyager 2J. Geophys. Res. 92, 14987-15001
Lindal, G.F. 1992, The atmosphere of Neptune - an analysis of radio occultation data acquired with Voyager 2 Astron J 103, 967-982
Lodders, K. 2004, Jupiter formed with more tar than ice. Astrophys. J. 611(1), 587-597
Lodders, K. and B. Fegley, B. 1998,'The Planetary Scientist's Companion,' Oxford University Press, New York, NY.
Mahaffy, P.R., Niemann, H.B., Alpert, A., Atreya, S.K., Demick, J., Donahue, T.M., Harpold, D.N., Owen, T.C., 2000, Noble gas abundance and isotope ratios in the atmosphere of Jupiter from the Galileo probe mass spectrometer. JGR Planets 105, 15061-15072
Menzel, D.H. 1923, Water-cell transmissions and planetary temperatures, Astrophys. J. 58, 65-74
Menzel, D.H. 1930, Hydrogen abundance and the constitution of the giant planets, Publ. Astron, Soc. Pacific, 42, 228-232
Owen, T. Mahaffy, P., Niemann, H.B., Atreya, S., Donahue, T., Bar-Nun, A., dePater, I 2001, A low-temperature origin for the planetesimals that formed Jupiter, Nature, 402, 269-270
Pollack, J.B., Hubickyj, O., Bodenheimer, P., Lissauer, J.J., Podolak, M., Greenzweig, Y, 1996, Formation of the Giant Planets by Concurrent Accretion of Solids and Gas, Icarus 124, 62-85
Seiff, A., Kirk, D.B., Knight, T.C.D., Young, R.E., Mihalov, J.D., Young, L.A., Milos, F.S., Schubert, G., Blanchard, R.C., Atkinson, D. 1998, Thermal structure of Jupiter's atmosphere near the edge of a 5-μm hot spot in the north equatorial belt, J. Geophys. Res., 103, 22857-22890
Slipher, V.M. 1909, The spectra of the major planets, Lowell Obs. Bull. 42, 231-238
Wildt, R. 1932, Absorptionsspektren und Atmosphaeren der grossen Planeten, Nachr. D. Akad. Wiss. Goettingen, Math. Phys. Kl. II, 87-96
Wong, M.H., Mahaffy, P.R., Atreya, S.K., Niemann, H.B., Owen, T.C. 2004, Updated Galileo probe mass spectrometer measurements of carbon, oxygen, nitrogen, and sulfur on Jupiter, Icarus, 171, 153-170